Venstra Medical’s heart pump promising better results for smaller patients – such as women – was among a raft of heart and diabetes companies to receive $12 million in grants last week.

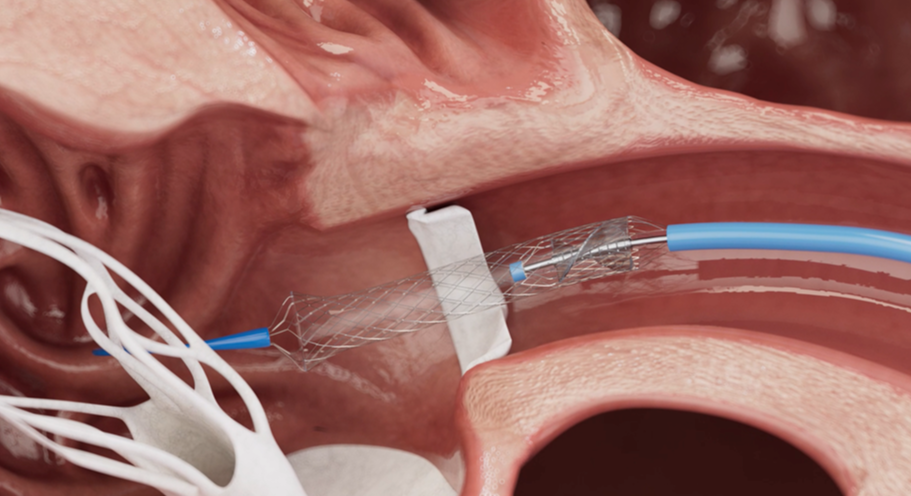
Australian start-up Venstra Medical has developed what it claims to be the world’s smallest and most powerful percutaneous ventricular assist device [pVAD] for treating “cardiogenic shock”—in which the heart suddenly can’t pump enough blood to meet the body’s needs.
As part of a fresh $12 million push to support cardiovascular and diabetes innovations in Australia [see below], not-for-profit biotech accelerator MTPConnect last week awarded Venstra Medical $1 million to develop its next-generation pump.
Venstra is aiming to disrupt a US$1.5 billion global market dominated by a single player, Abiomed, which Johnson & Johnson bought for US$17 billion in 2022. Abiomed’s singular focus is its Impella range of heart pumps that are inserted into the left ventricle to keep the heart going during operations or during cardiogenic shock
There are about 100,000 cardiogenic shock cases a year in the US and it is fatal in between 30% and 50% of them. But for women, mortality is 10% worse.
“It’s the biggest market that no one’s ever heard of.”
Venstra Medical CEO Martin Cook
Cardiogenic shock is most often caused by a severe heart attack but can have other causes.
The grant from MTPConnect’s Targeted Translation Research Accelerator (TTRA) program is the latest validation for the founders, who’ve already raised $8 million from private investors and government schemes.
Venstra Medical is not the only one taking on the Abiomed monopoly. In March, California-based Supira Medical, with a portfolio of devices in trials, announced an oversubscribed Series E financing round of US$120 million.
Last year, a trial published in the New England Journal of Medicine showed Abiomed’s Impella improved survival by 13%, but had four times more complications than if no pump was used. The complications, however, “did not overshadow the benefits” of the device.
Venstra has developed a collapsible, expandable device it says could save more lives, especially women’s – developed in laboratories spanning Sydney, Newcastle [Australia] and the Mayo Clinic in Minneapolis, where Australia CEO Martin Cook now lives.
Venstra was founded in 2018 by engineer Cook and Newcastle-based cardiologist and inventor Dr Sukumaran Thambar. They had just sold their previous heart-valve-tech venture, Percutaneous Cardiovascular Solutions, to California-based medtech company Edwards Life Sciences for $4 million. Its IP has since been incorporated into Edwards’s devices.
Thambar says that if they’d been able to get their mitral valve replacement into human trials, “the company would have been worth hundreds of millions”. Australia’s then immature venture scene and the global financial crisis had proved too difficult to overcome. “But it gave us a track record of successful developments and has helped us in our current journey,” Dr Thambar tells Forbes Australia.
After the sale, they wondered what was next. Cardiologist Thambar – who’d been fascinated by the heart since his mother had a heart attack when he was young in Sri Lanka – had used an Impella device during a brief period in which they were available in Australia through a distributor. The FDA had approved it in the US in 2015 and they could see it was saving lives – and doing good business – but it had limitations.
The larger version had more power but caused more damage squeezing into the veins. The smaller version was less intrusive but didn’t have enough pumping power.
Abiomed’s Impella is often too large for female arteries, Professor Chris Hayward, a transplant cardiologist at St Vincent’s Hospital Sydney, told Forbes Australia.
A secondary analysis of the trial data, however, suggested that the survival disadvantage of women was due to the women in the study being older and receiving the device later. Other studies have shown women to have a better survival rate with Impella, but a worse complication rate possibly due to the size of their arteries.
Johnson & Johnson spokesman Alexander Zolbert said the Impella had been used in more than 350,000 patients.
Cook scanned the academic literature and the US patent office and found a portfolio of patents gathering dust since the global financial crisis.
“We licensed it. And we’ve created a lot of our own IP,” says Cook. “We’ve developed a next-generation technology which is the best of both worlds for size and power, and it’s based on a collapsible-expandable approach. It’s the world’s smallest blood pump.
“Imagine you’ve got a patient who has either suffered a massive heart attack or they’re at end-stage heart failure. They get rushed into the cath lab. You’ve got minutes to hours to do something for the patient or else they’re going to die. So it’s not this long-term heart failure thing that a company like [Australian-founded permanent heart-pump company] Bivacor treats.
“We put in our device … It’s only three millimetres in diameter.” The catheter is fed from the femoral artery up into the heart. “And they put it across the aortic valve … and our device pops into shape and starts pumping.”
External funding came from an Australian Federal Government Biomed Horizons grant in 2020. Cook raised a seed round from Australian angel investors, then raised a further $8 million in 2022.
“This is an expensive business,” says Cook. “We’re looking to raise a big chunk to get us to where Bivacor is at now, having done a few patients. But ours is an even bigger opportunity than Bivacor, I would say.
“The one player in our field, Abiomed, all they do are these PVAD devices.”
While the Impella pulls in US$1.5 billion a year, Abiomed’s $17 billion valuation hinged on anticipated further uses for it, including treatment of acute ST Elevation Myocardial Infarction [STEMI]. In Australia, Abiomed’s Impella is in use only in a limited number of centres but its use received government funding last year.
“It’s the biggest market that no one’s ever heard of,” says Cook. “And there’s another use. Often you have patients who need a stent implanted because they’ve got a coronary artery block. It’s not an emergency case, but they’re high risk for some reason. Maybe they’re old, maybe they’ve got some other comorbidity.
“So they put in one of these pumps during the operation to take over from the heart because there’s a good chance that the heart will stop during the procedure.”
While the Impella catheter is 5mm wide, Venstra’s is 3mm and pumps twice the volume at a higher pressure, says Thambar.
Cardiologist Professor Chris Hayward said that 2mm reduction is crucial, and he’s looking forward to testing the Venstra device.
“The femoral artery is between 3mm to 7mm, so if you’ve got a 3mm artery then that’s obviously too small for an Impella. You can probably stretch it up to 4mm but that’s where the complications come when you’re really at the limit of your access.” Small women, particularly Asian women, stood to benefit most, said Hayward who is on the Venstra medical advisory board. “They tend to have a smaller vascular dimension. So that’s a real-world issue.”
But Venstra has only been tested in animals to date. Cook wants the current fundraising round to take them through to first-in-human trials which, depending on the timing of the raise, would begin in the first half of 2026 in NSW.
“That’s going to be in those high-risk patients that only get the device for an hour or two during a procedure. Then we’re going to move on to a cardiogenic shock study which will be the exciting part. That’s a really big value inflection point.”
So having sold one company, are the founders open to selling Venstra Medical?
“We’ve spoken to three major med-tech strategic companies, like the Medtronics of this world,” says Cook. “We’ve had three visits, including due diligence teams and their engineers, come out to see us. So it’s been close, but we’re just a little bit early. They tend to want to buy something when it’s at later stage and is a bit more de-risked.”
In the meantime, Thambar will keep using the Impella which only came back to Australia in the last few years. “One of the patients that I put the device on survived the heart attack and we got them down to St. Vincent’s, and he ended up with a heart transplant. So it’s definitely useful and available, but it can be made better.
“Australia is batting well above its weight in the area of heart pump development,” Thambar says. “We have a history of heart pumps. Most recently it’s Bivacor. It will be useful for a handful of patients every year. But this will be for hundreds of patients every year.
“Some of the patients we save will require a transplant or a Bivacor.
“And the ecosystem for developing these things has changed in Australia in the last 20 years. Last time, [with his previous startup] we were very early, and we didn’t have all the grants and all the support that we currently have. These things help. They help you prototype. They help you to have some data when you go to investors.
MTPConnect announced the new funding today at the BIO International Convention in Boston, one of the world’s largest biotech events.
MTPConnect CEO Stuart Dignam said such investment was critical to getting Australian
innovations off the ground.
“Access to funding remains the biggest challenge for start-ups and innovators,” Dignam said. “The capital markets, especially when you’re at an early stage with your innovation, are particularly tough – and now they have heightened global business uncertainty and tariff turmoil to contend with.”
MTPConnect is a not-for-profit life sciences accelerator championing the growth of Australia’s medical products sector. It has delivered $47 million in its Targeted Translation Research Accelerator program for diabetes and cardiovascular since 2020 on behalf of the Medical Research Future Fund (MRFF).
Targeted Translation Research Accelerator grants
Company | State | Funding | Project |
|---|---|---|---|
Anaxis Pharma Pty Ltd | VIC | $333,236 | Targeting cell death in diabetic kidney disease |
Argenica Therapeutics Limited | WA | $1,000,000 | Phase 2b/3 adaptive trial to determine the safety and efficacy of ARG-007 in reducing disability in acute ischaemic stroke patients |
Aspecthera Pty Ltd | TAS | $500,000 | Novel eye-drop therapy for non-proliferative diabetic retinopathy to prevent vision loss and disease progression |
Atherid Therapeutics Pty Ltd | WA | $750,000 | Establishing GMP production of a biologic therapeutic for treating atherosclerotic cardiovascular disease |
CathRx Ltd | NSW | $1,100,000 | ElectroPulse pulsed field ablation system to treat atrial fibrillation |
Endo Axiom Pty Ltd | NSW | $1,079,424 | Evaluating oral insulin for type 1 diabetes |
I D & E Pty Ltd | NSW | $1,200,000 | Ocular drug delivery system |
Inosi Therapeutics Pty Ltd | VIC | $968,192.63 | Development of an IRAP inhibitor for the treatment of diabetic kidney disease |
Nanomedx Pty Ltd | NSW | $750,000 | Local regulation of inflammation for the treatment of peripheral arterial disease |
ProGenis Pharmaceuticals Pty Ltd | WA | $369,706 | PGP-011 as an effective RNA therapeutic for improving insulin sensitivity |
Theia Medical Pty Ltd | SA | $1,000,000 | Hybrid 3D-printed intravascular imaging device for accurate detection of high-risk coronary artery disease |
Venstra Medical Pty Ltd | NSW | $1,000,000 | The world’s lowest-profile, most powerful temporary heart pump for patients in cardiogenic shock |
Wavewise Analytics Pty Ltd (formerly Cyban) | VIC | $996,995 | Development of the ARGUS system: real-time, non-invasive blood flow monitoring for stroke detection |
ZiP Diagnostics Pty Ltd | VIC | $1,019,134 | Development of a low-cost, rapid, point-of-care test for prediction and early diagnosis of preeclampsia |



