Saluda had a disastrous debut on the ASX on Friday, shedding half its value. But here’s the inside story of resilience in the company that learnt how to read signals going up the spinal cord and to turned them into pain relief.

Saluda founder John Parker has just delivered a talk at an international biomedical engineering conference, and a line of fanboys and girls forms in front of him.
He’s telling a guy in skinny jeans and a sports jacket: “People in the US talk about having been through three failed start-ups, like it’s something to be proud of … No! Get into someone else’s start-up. Learn as much as you can, then get out. Do it on someone else’s money.”
A young woman with long red hair says she has an idea for a medical device and wants to know a ballpark figure for what it costs to get to in-human trials. “$10 million,” he tells her without pause. “I don’t know why, but they all seem to come out about there.” One has a question about neuroscience, another about FDA approval processes.
Happens all the time, Parker says later. It’s hard for his brain to move between all the realms in which he has succeeded. He usually gets kicked out of the auditorium because the next session is about to start.

The fact is, Parker has jumped through more hoops than most, and with a mix of idealism and pragmatism bordering on cynicism, he’s done what few Australians have achieved, taking a blue-sky idea born in a government lab and getting it to market. And now to and ASX IPO that will raise $231 million and valuing the company at around $775 millioin.
Over the decade in which the world grappled with a pain epidemic, it raised half a billion US dollars to build the first “closed-loop” spinal cord stimulator for chronic pain – a system that reads the body’s own electrical signals and adjusts itself in real time.
With Parker gone, and a new regime in charge, Saluda is scaling up its US ambitions and taking on the giants in medtech. But it all started with a Howard government scheme to ramp up communications technology and a physics graduate acting like an engineer.
The impostor
John Parker has been suffering from impostor syndrome from the start of his career. For his PhD in physics at the Australian National University, he’d built an instrument that measured “intermolecular forces between molecules and surfaces.”
The ANU commercialised it because companies like IBM, Xerox and Kodak wanted to understand what was happening in the ever-shrinking worlds they needed to work within.
“I could have a lot of fun with $380 million of research.”
Then NICTA chief David Skellern
“I got a royalty from it,” says Parker. “It kinda bought our first house (in Canberra), which is pretty good for a 25-year-old kid.”
The university sent him around the world as the device’s salesman. “I had a lot of fun.”
He spent a few years in Sweden doing further research on it when, in 1993, he got a call from a headhunter representing the nascent start-up Cochlear. “I nearly fell off my chair. Nobody ever called my phone. I’d never been called by a headhunter before. I had no skills in that area.”
But academics were having it drummed into them that research needed to turn a quid. Parker had been paraded as the paragon of this new paradigm. “That’s how Cochlear knew about me,” he says.
He took the Cochlear job, figuring he’d do it for a few years before getting a real job back in academia. “Cochlear was transitioning from the first product. It wasn’t really scalable. It was more or less a prototype that had leaked out into the market. I ran the team that developed the next-generation plan. I had no background in it. I’d wake up at night paranoid that they’d find out I didn’t have a degree in engineering, then spent the rest of my career doing engineering.”
The then-Cochlear CEO, Catherine Livingstone, was a big supporter and mentor, he says. “She moved me around the company, doing everything except sales and marketing. Cochlear floated in 1995. The company did very well out of the float. My options were very good, so I managed to buy another house. In Roseville [Sydney].”
Parker ended up as Cochlear’s chief technical officer and a board member, but the arrival of Chris Roberts as CEO in 2004 signaled the end for him.

“We just had a different view of where the company should go. Chris was very much focused on vertically integrating and buying distributors, and I was more interested in diversifying the product, both outside the ear and within it.” Not wanting to be the stick in the mud at every board meeting, Parker, aged 42, retired.
A big step
He’d been retired for three days in 2006 when the phone rang again. It was Dave Skellern, CEO of NICTA (the National Information and Communications Technology Agency), a Howard Government scheme to promote information and communications training at PhD level.

Skellern had been one of the key players in spinning WiFi out of the CSIRO into the company Radiata, which was bought by Cisco Systems in 2001 for $565 million. Skellern had also thought he’d retire after leaving Cisco in 2004, but the offer to be CEO of NICTA with a $380 million budget was hard to refuse.
“Just do something really cool.”
Dave Skellern, then CEO of NICTA
“I could have a lot of fun with $380 million of research,” he recalls thinking.
Skellern wanted to orient NICTA towards moonshot projects. Perhaps its biggest success was the OKL4 microkernel, which now runs in more than 2 billion mobile devices. Another was the ASX listed Audinate, now worth $358 million, but which at its peak last year was almost a $2 billion company.
Skellern knew Parker and got him to consult on a few projects, but Parker wanted to hold the line on his retirement. “He kept offering me other projects,” says Parker. “And I kept saying no. But eventually he came to me and he said, ‘How about you tell me what you want to do, and I get you the money for it?’”
Parker asked what the boundaries were. “Just do something really cool.”
For his part, Skellern recalls asking Parker to work on a bionic eye, but Parker had his doubts about that working and qualifying for reimbursement.
“Well, what do you want to do?”
“I’ve got this idea. I’ve had it for ages.”
Parker had wanted to do it at Cochlear, where he had learnt about stimulating nerve cells to send sound signals to the brain. The idea he put forward to Skellern was that they should try that for other nerves, other signals. Maybe pain.
Parker was determined that, since there was a bucket of money with no need for a return, they should reach for the stars. “A lot of research is risk averse. It didn’t matter whether or not this was successful, so I figured we had to take a big step.”
Eureka!
Dean Karantonis was a software guy with a master’s in biomedical engineering working at Ventracor, an ill-fated heart-pump start-up in Sydney, when he got the call from Parker. He joined Parker’s team at NICTA on the strength of Parker’s reputation. They were still figuring out what to do. It was Parker’s pragmatism on top of his idealism that pushed them into pain, says Karantonis.

“We started to go down the path of looking at spinal cord stim for chronic pain because it was a large and established market where you could get reimbursed already,” says Karantonis. “Which means you’re going to get a return on investment if you’re finding out something interesting. That was one of John’s big strategic decisions.”
So, if they started with a really difficult problem – measuring nerve signals in the ever-moving spinal cord – with a “big reimburse market”, then maybe down the track they could apply the technology in other areas of the body.
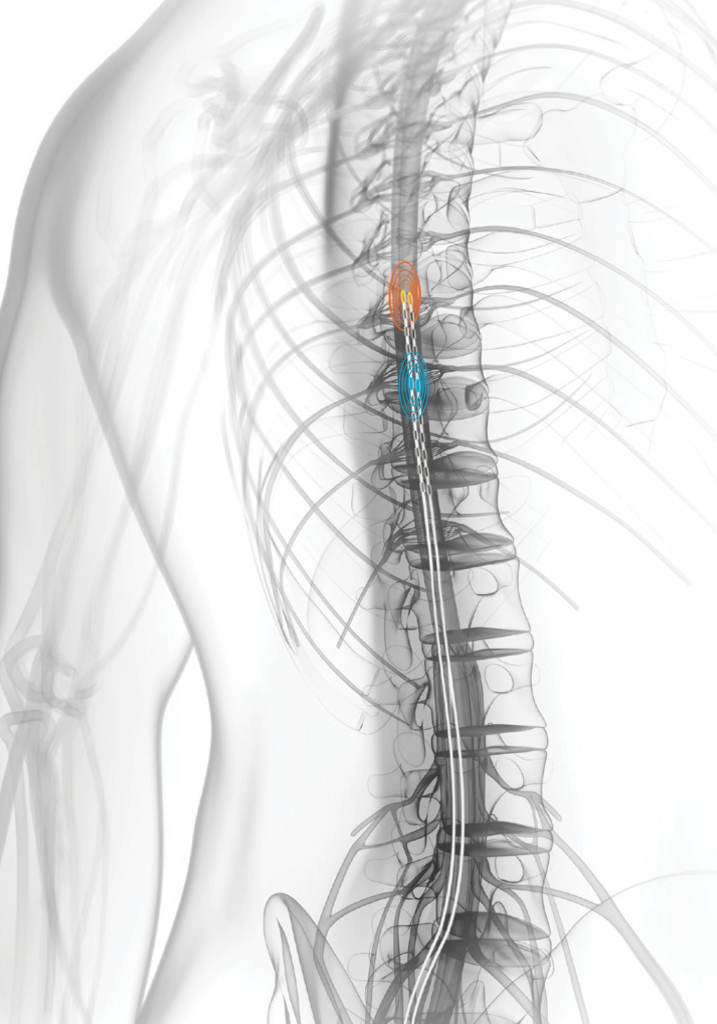
People had been walking around with spinal cord stimulator wires in their backs since the 1960s. Spinal cord stimulators work by delivering mild electrical pulses to the spinal cord through a small device implanted under the skin. These pulses interfere with pain signals before they reach the brain.
The problem, as Parker saw it, was that the spine moves. And with each movement, the wires moved away from, or closer to, the nerves. Patients were often over stimulated or under stimulated.
When people were overstimulated, they felt a tingling sensation, and if that got too strong, they usually turned the device down or off, so they lost any benefit.
Parker thought they could develop a system that measured the amount of stimulation received in each pulse and adjust it immediately – a “closed-loop” system.
The first thing to do, recalls Karantonis, now senior vice president of therapy development at Saluda, was to figure out why no one had done this before.
“It’s an obvious thing to try. Cochlear had done it for hearing. But we quickly found out that it was a really difficult technical problem. You’re like a pacemaker. You’re injecting electrical pulses into the body, and we’re trying to sense the response of your nervous system to those pulses very quickly after that pulse is delivered. And they’re tiny. The signals we’re measuring are about a million times smaller than the pulses we’re outputting.
“But John got the right skill set in the room and let us run for a few months to solve that core technical problem. And with enough time and dedication, we did.”
The eureka moment came in early 2010 when they hooked up a sheep. They stimulated its nerve cells and then were able to measure the signal sent back to that nerve by the brain in response to that stimulation.
“The benefits of doing this work as you get older are that you realise you’re in a pivotal moment,” recalls Parker. “When we started seeing measurements popping up on the screen, the pieces quickly fell into place, and it was obvious it was going to be important.”
By the end of 2010, they’d measured the same signal in a human.
The Big Bang
In four years at NICTA, they only published two papers. Skellern had thought publication was just a bonus. “We were about creating IT-based systems that would give an Australian company a competitive advantage in the marketplace. We wanted to solve all the world’s big problems.”
But Skellern’s successor as head of NICTA, Professor Hugh Durrant-Whyte, saw the project’s lack of publications as a failure, says Parker.
Saluda’s first money came in the form of $5 million as the first grant of the NSW Medical Devices Fund, plus $5 million from the Kinghorn Foundation – set up by John and Geoffrey Kinghorn after their float of RAMS Home Loans.
But Saluda was going to need a lot more.
When Greg Plamondon first walked into the Saluda office in 2014, he thought he was walking onto the set of The Big Bang Theory. He was just back from doing six years in Denver for Cochlear as their vice president of finance in the US when Parker had called. “Hey, I want to tell you some interesting work we’re doing.”

Plamondon visited their then Redfern office and was hooked. “I think I was the first non-scientist/engineer to join,” he says. “The energy was contagious. People were doing long hours, long nights, but there was just a passion to make a breakthrough.”
At first, Plamondon found it hard to raise money without clinical data, but that was on the way.
The Doctor
When Australia’s “doyen of pain medicine,” Professor Michael Cousins, phoned Dr Marc Russo in 2013, the message was clear: “I need a young buck to help these guys produce a better stimulator.” Pain specialist Russo didn’t hesitate.

Soon after, Russo met Saluda founder John Parker — and was struck by how different he was from the slick American CEOs he’d known. When Russo asked how long it would take to bring the technology to market, Parker replied, “I have no idea. As long as it takes to do the science right.” That, says Russo, was why he signed on.
The very first spinal cord stimulation devices were implanted in the year Russo was born, 1967, and most of the work over the next 40 years was put into getting the hardware right, he says. “The device often produced quite dramatic pain relief. But by the fourth or fifth or sixth year, the patients were telling us that their pain was coming back.”
In human
Still a long way from having an implantable device, Russo recalls wheeling in a suitcase-sized box of knobs and gauges, and hooking it up to a patient who had the leads from another company’s spinal cord stim device sticking out of their back.
Upon having the Saluda device turned on, Karantonis remembers seeing that first signal show up on the dials. “It’s not too many times in your career you’ll get a chance to come up with that moment of discovery,” he recalls.
And as far as the patient went … “They thought it was better than the current state-of-the-art stimulator,” says Russo.
Plamondon, now Saluda’s chief operating officer, suddenly found his job of raising money got a lot easier. Melbourne-founded BioScience Managers led the US$10 million Series B round in February 2015, and it was time to get it into humans more permanently.

Less than two years later, Russo was inserting Saluda devices in the first major trial. Seventy patients had a device in for a week with an external battery, and 50 agreed to have a battery inserted under their skin for the long term.
Wonders
When Peter Todd’s pain doctor suggested he take part in a trial that would involve having wires stuck into his spinal cord, he wasn’t convinced. “Mate, I don’t need wires in my back. I need the pain to stop.”

But Todd was desperate. Since falling off the back of an Army truck in 1982, the former soldier had endured steadily rising agony. Surgery hadn’t helped. One doctor had him on methadone for three years, but Todd knew his growing addiction was a dead end.
And so he agreed to give the device a try. For a week, he walked around with a battery pack dangling from his back. He fiddled with it, turning it up and down, feeling a weird tingling down his legs.
He tested himself. He mowed the lawn and pulled some weeds. “I found I could do things around the house and in the yard that I couldn’t do before,” he says.
But the big test was sleep.
“One night, I taped the battery to my skin, and I had the best night’s sleep. I had no pain, no nothing.” At the end of the trial week, he hadn’t used any of the painkillers they’d given him.
In November 2016, Todd became the 50th participant in Saluda’s first clinical trial. They inserted a permanent battery at the top of his buttocks, and nine years later, it’s due to be replaced. It’s allowed the now 63-year-old to move onto a five acre block at Brookfield, 200 km north of Sydney.
“As I’m getting older, other things are starting to play up. My knees are starting to go, the next lot of vertebrae are going, but [pain doctor] Nathan [Taylor] doesn’t believe in painkillers.” He wishes he could get a similar device for his other parts.

Todd can’t imagine what his life would be like without the device. “I’d probably have a rod in my back and be addicted to painkillers. Mine will stay in until I die because it’s doing wonders for me.”
Like Todd, many of the original patients still have the devices, says Dr Russo. “What we’ve seen is that the loss of effect over time no longer occurs. So it looks like we’ve cured that problem. At nine years, they’re still going strong. They’re still giving pain relief day by day by day by day.
“We used to say to patients getting spinal cord stimulation that there’s a 50% chance you’ll get 50% or more pain relief. We used to call it the 50-50 club. They were in such pain, they were happy to take those odds.”

The Australian study, on which Russo was the lead author, found 77% of patients got better than 50% pain reduction, while 56% got more than 80% pain reduction. That was enough to get the device approved in Australia and Europe.
The US FDA required a larger randomised controlled study over three years. Published in 2022, it got even better results, and Saluda’s Evoke device was approved for US sales that year.
“The next port of call is the 80-80 club,” says Russo, “where 80% of patients are getting 80% to 100% pain relief. I’m committed to that. I want to see that before I retire.”
Russo has active partnerships with multiple spinal cord stimulator companies to help develop their devices. “I’ve invented my own waveform called Subwave. I did not patent it. I gave it away and had it as an open resource. So that waveform is now able to be put into every company’s device.”
While Saluda was first to market with a closed-loop system, the rest of the field has been scrambling to catch up. Giants like Medtronic, Abbott, Boston Scientific, and Nevro have all poured resources into developing their own versions, but Russo says Saluda’s “deep patent moat” makes it hard to replicate the technology directly.

“They’ve got to do all that same really hard stuff that Saluda already had to do,” he says.
For decades, the spinal cord stimulation market grew steadily – around 10% a year – as chronic pain became one of the world’s fastest-rising health burdens. But a series of studies in recent years cast doubt on the efficacy of such devices.
Russo says the studies were flawed and, besides, they were looking at the old systems. “Back pain is an absolute epidemic. The number of spine surgeries keeps increasing, and 70% of spinal cord stimulators are implanted because of the outcomes of those [failed] surgeries.”
Russo is working with many different companies in the space. And while Saluda is out in front now, Russo expects the field to keep evolving. “We’ll get new treatments, maybe things that mean we won’t need stimulators for everyone,” he says. “But right now, they’ve never been more effective – and we’ve only just scratched the surface of what’s possible.”
Crunching some numbers
Saluda generated US$70 million in revenue in the year to June 2025.
Management says that will grow to US$82 million this financial year.
Saluda has 120 US salespeople supporting about 250 doctors.
It lost US$128 million in the year to June 2025 and is forecast to lose US$145 million this year.
Saluda values the global opportunity at US$23 billion.
John Parker’s rules for building a biotech

Superheroes don’t exist: Get a lot of people to work really hard for a long time to solve many problems. You solve them by grinding them to dust. What you want instead of Superman is Worksalot Dude.
Life is short, choose wisely: Choose the one idea you can get to work.
Read the map: “We looked very carefully at the patent literature. There were 2,500 patents on spinal cord stimulators. Patents are terrific because they tell you what competitors think five years before they make a product.”
Science the shit out of it: “We built five iterations of the hardware. So by the time we decided to build an implant and develop a therapy, we already had clinical data from a number of different animals and about 100 human subjects.”
If you get into trouble, ask an adult.



